Deep beneath the earth in Naica, Mexico, lies a geological marvel that defies imagination – the Giant Crystal Cave. Discovered in 2000 by miners seeking new ore deposits, this hidden chamber is home to some of the largest natural crystals ever found. Towering, milky-white gypsum crystals, some as long as telephone poles, fill the cave, creating a breathtaking spectacle of natural artistry. These colossal formations, far exceeding anything seen in ordinary caves, have captivated scientists and nature enthusiasts alike, making the Giant Crystal Cave Mexico a subject of intense fascination and study.
For nearly two decades, researchers from around the globe have ventured into the oppressively hot and humid environment of the giant crystal cave mexico to unlock the secrets of its formation and growth. Crystallographers, mineralogists, and geologists have been drawn to this subterranean wonder, eager to understand the unique conditions that allowed such enormous crystals to develop. Their investigations have not only unveiled the cave’s origins but have also raised important questions about its preservation for future generations. The giant crystal cave mexico is more than just a beautiful sight; it’s a natural laboratory offering invaluable insights into geological processes and crystal growth on a grand scale.
The Birthplace of Giants: How the Crystal Cave Formed
The story of the giant crystal cave mexico begins millions of years ago, approximately 26 million years, when magma surged upwards through the Earth’s crust in southeastern Chihuahua, Mexico. This geological upheaval created the mountain near Naica and infused the surrounding limestone with hot, mineral-rich waters. These heated waters, saturated with calcium sulfate, seeped into underground caverns, setting the stage for the extraordinary crystal growth that would follow.
Within the giant crystal cave mexico, the dominant mineral that precipitated out of the calcium sulfate-rich water was gypsum (CaSO4·2H2O), specifically selenite, a transparent and colorless variety. The chemical structure of gypsum is unique, composed of calcium sulfate layers interspersed with double layers of water molecules. This structure plays a crucial role in its formation and growth.
 Photgraph shows a person in the Cave of Crystals with giant white gypsum crystals criss-crossing behind him.Credit: Javier Trueba/MSF/Science Source
Photgraph shows a person in the Cave of Crystals with giant white gypsum crystals criss-crossing behind him.Credit: Javier Trueba/MSF/Science Source
A visitor marvels at the immense gypsum crystals within the Giant Crystal Cave of Mexico.
Crystallographer Alexander Van Driessche, from the National Center for Scientific Research’s Institute of Earth Science, explains that the stability of minerals like gypsum and anhydrite (CaSO4) is temperature-dependent. Anhydrite is more stable at temperatures above 58 °C, while gypsum is more stable below this threshold. Initially, in the hot, magma-heated waters of the giant crystal cave mexico, anhydrite deposits formed. Over vast stretches of time, as the water gradually cooled below 58 °C, the anhydrite began to dissolve. This dissolution process released calcium and sulfate into the solution, creating a slightly supersaturated environment ideal for the nucleation and slow growth of gypsum crystals. This precisely balanced process was key to the formation of the massive crystals in the giant crystal cave mexico.
Nucleation and the Infinitesimal Pace of Growth
Crystal growth, whether in a laboratory or a natural cave like the giant crystal cave mexico, always begins with nucleation. This is the initial stage where molecular building blocks arrange themselves around a tiny nucleus and start to form a crystal structure. In solutions with high supersaturation, numerous crystals nucleate but tend to remain small. Conversely, low supersaturation favors fewer nucleation sites but allows for significant crystal growth. The giant crystal cave mexico provided the perfect conditions for slow nucleation and exceptionally prolonged growth, resulting in the gigantic selenite crystals we see today.
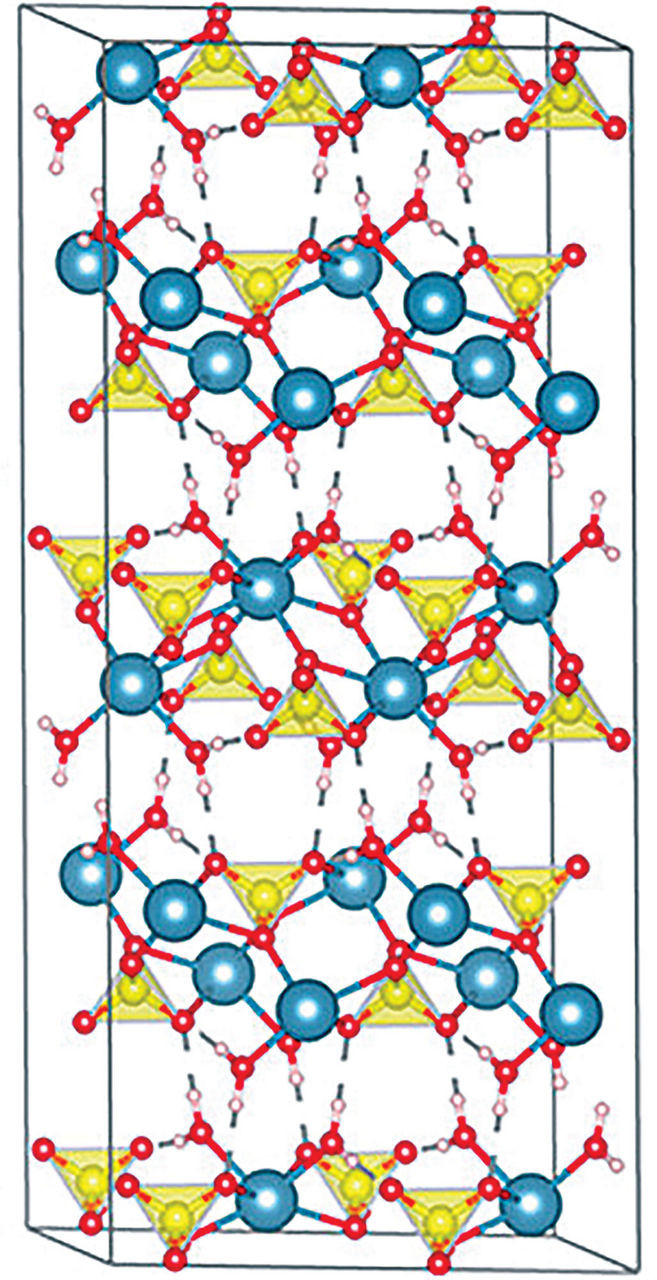 Image shows the crystal structure of gypsum.Credit: Cryst. Growth Des.
Image shows the crystal structure of gypsum.Credit: Cryst. Growth Des.
Intrigued by the scale of the crystals in the giant crystal cave mexico, Van Driessche and his colleagues conducted laboratory experiments to study gypsum nucleation. Their findings revealed an unconventional nucleation process: gypsum crystals in the giant crystal cave mexico appear to form from coalescing nanoclusters of CaSO4, rather than from the traditional formation around a tiny gypsum seed crystal. This discovery shed new light on the fundamental mechanisms of crystal formation.
Beyond nucleation, the sheer size of the crystals in the giant crystal cave mexico begged the question: how did they grow so large? The answer lies in the incredibly slow, almost imperceptible rate of growth over immense timescales.
A Tale of Two Caves: Cooling Rates and Crystal Size
Interestingly, the giant crystal cave mexico is not the only cave in the Naica mine containing gypsum crystals. The Cave of Swords, located at a shallower depth of 120 meters, also features gypsum formations. However, the crystals in the Cave of Swords are markedly different – smaller and sword-like, coating the cave walls in a dense array of crystals up to 2 meters long. This contrast provides a crucial clue to understanding the exceptional size of the crystals in the giant crystal cave mexico.
 Image shows a person in the narrow Cave of Swords with swordlike crystals covering the walls on both sides.Credit: Javier Trueba/MSF/Science Source
Image shows a person in the narrow Cave of Swords with swordlike crystals covering the walls on both sides.Credit: Javier Trueba/MSF/Science Source
Compared to the Giant Crystal Cave, the Cave of Swords in Mexico features smaller, sword-like gypsum crystals due to faster cooling rates.
The key difference between the two caves is the rate of cooling. In the Cave of Swords, the water temperature cooled down more rapidly than in the deeper giant crystal cave mexico. This faster cooling favored nucleation over crystal growth, resulting in a multitude of smaller crystals. In the giant crystal cave mexico, the water temperature remained within the narrow temperature range conducive to gypsum formation for an extraordinarily long period. This incredibly slow and gradual cooling meant that only a few gypsum crystals nucleated, but these few had the luxury of almost limitless time to grow to their colossal dimensions.
To determine the age of these crystal giants, researchers painstakingly measured crystal growth rates in the lab using water samples from the Naica mine. By employing phase-shifting interferometry, a highly sensitive light-based technique, they could measure surface growth rates as minute as 10–5 nm/s. These measurements, taken across the temperature range relevant to the giant crystal cave mexico (54–58 °C), allowed them to estimate how long it would take a 1-meter-thick crystal to grow at 55 °C.
The answer was astounding: approximately 1 million years. This mind-bogglingly slow growth rate translates to adding only the thickness of a sheet of paper every 200 years. This revelation underscores the incredible patience of geological time and the unique, stable conditions that persisted in the giant crystal cave mexico for millennia.
A Fragile Wonder in a Hostile Environment
The mining operations at Naica, while leading to the discovery of the giant crystal cave mexico, have also inadvertently created challenges for its long-term survival. To access ore deposits, the mining company Industrias Peñoles has been pumping water out of the mountain, lowering the water table and exposing the caves to air. While this drainage allowed for the exploration of the giant crystal cave mexico, it also introduced a harsh and potentially damaging environment.
The conditions inside the giant crystal cave mexico are extreme, pushing human endurance to its limits. Temperatures reach a scorching 50 °C (122 °F) with a relative humidity exceeding 90%. In this environment, sweat provides no cooling relief, and researchers could only endure short 10–15 minute visits, even with medical checks and protective gear.
Beyond the challenges for human visitors, the drained environment poses a threat to the crystals themselves. Weighing an estimated 40–50 metric tons each, the massive crystals in the giant crystal cave mexico are no longer fully supported by buoyant water. This lack of support increases the risk of cracking under their own immense weight. Furthermore, gypsum is a soft mineral, and even the foot traffic of researchers has begun to wear a visible path into the crystal floor.
Researchers have been studying the effects of dehydration on the crystals in the giant crystal cave mexico. Laboratory experiments subjecting crystal samples to gaseous environments have revealed the formation of bassanite, a dehydrated form of calcium sulfate, on the crystal surfaces. This indicates that the crystals are indeed undergoing changes due to the drier conditions, potentially affecting their long-term appearance and integrity. Removing the crystals from the cave for preservation is not considered a viable option, making in-situ preservation efforts crucial.
An Uncertain Future and Lingering Mysteries
Mining operations near the giant crystal cave mexico have been suspended since around 2015 due to flooding in the mine. While the water level has risen, it remains uncertain whether the giant crystal cave mexico itself will be submerged again. The future of this extraordinary geological site hangs in the balance.
 Photo shows a person inside a cave containing large, clear gypsum crystals.Credit: Javier Trueba/MSF/Science Source
Photo shows a person inside a cave containing large, clear gypsum crystals.Credit: Javier Trueba/MSF/Science Source
While not as large, the gypsum crystals in Pulpí, Spain, offer another site for studying crystal formation, compared to the Giant Crystal Cave of Mexico.
Despite the uncertainties surrounding the giant crystal cave mexico, research into gypsum crystallization continues elsewhere. Scientists are exploring other gypsum-rich locations, such as the crystal geode in Pulpí, Spain, to further unravel the mysteries of crystal growth and morphology. These studies, while not in the giant crystal cave mexico, contribute to our broader understanding of these fascinating geological formations.
For researchers like María Elena Montero-Cabrera, who has studied the preservation of the crystals, a return to the giant crystal cave mexico would be a welcome opportunity. The allure of this hidden, otherworldly beauty remains strong. Until access is potentially restored, the giant crystal cave mexico stands as a testament to the slow, powerful forces of nature, a hidden wonder awaiting an unknown future, and a compelling example of the geological treasures Mexico holds.