Deep beneath the surface of Naica, Mexico, lies a geological marvel that defies imagination – the Cave of Crystals, or as it’s known in Spanish, Cueva de los Cristales. This breathtaking cavern, filled with colossal gypsum crystals, presents a spectacle of natural artistry unlike anywhere else on Earth. Discovered in 2000 by miners seeking new ore deposits, this hidden world quickly became a focal point for scientists and nature enthusiasts alike, drawn to its rare beauty and the scientific enigmas it holds.
 A person stands in the Cave of Crystals amidst giant gypsum formations.
A person stands in the Cave of Crystals amidst giant gypsum formations.
Imagine stepping into a realm where translucent beams of gypsum, some stretching up to 12 meters in length and a meter in width, dominate the landscape. These massive, milky-white crystals, larger than telephone poles, jut out in every direction from the cave’s limestone walls, floor, and ceiling, creating an awe-inspiring, almost otherworldly scene. For crystallographer Juan Manuel García-Ruiz, who journeyed from Spain to witness this phenomenon, it was a career highlight. He described his initial experience as “euphoric,” a sentiment shared by many who have had the privilege to witness this subterranean wonder.
Nestled 290 meters below a mountain rich in lead, zinc, and silver, the Cave of Crystals owes its existence to the unique geological history of the Naica region. Since its unveiling by the mining company Industrias Peñoles, this subterranean chamber has become a magnet for global researchers. They are captivated not only by its extraordinary beauty but also by the scientific mysteries surrounding the origin and growth of these gigantic crystals. For nearly two decades, scientists have ventured into the cave’s intensely hot and humid environment, driven by the desire to unravel the secrets of its formation. Now, with many of the initial questions answered, the focus is shifting towards the crucial task of protecting and preserving these natural treasures for generations to come, a challenge made complex by the ongoing activities within the mine and the mountain itself.
The Birthplace of Giants: An Underground Flask
The story of the Mexican Crystal Cave begins around 26 million years ago when a surge of magma pushed upwards through the Earth’s crust beneath what is now southeastern Chihuahua, Mexico. This geological event led to the formation of the mountain near Naica and infused hot, mineral-rich waters into the caverns and fissures within the mountain’s limestone structure. It was within these mineral-laden waters that the remarkable crystals of Naica began their slow journey to becoming giants.
The cave was essentially a submerged chamber filled with water saturated with calcium sulfate. While calcium sulfate can crystallize into various minerals, in this unique environment, gypsum (CaSO4·2H2O), specifically the selenite variety – a transparent and colorless form – became the predominant mineral.
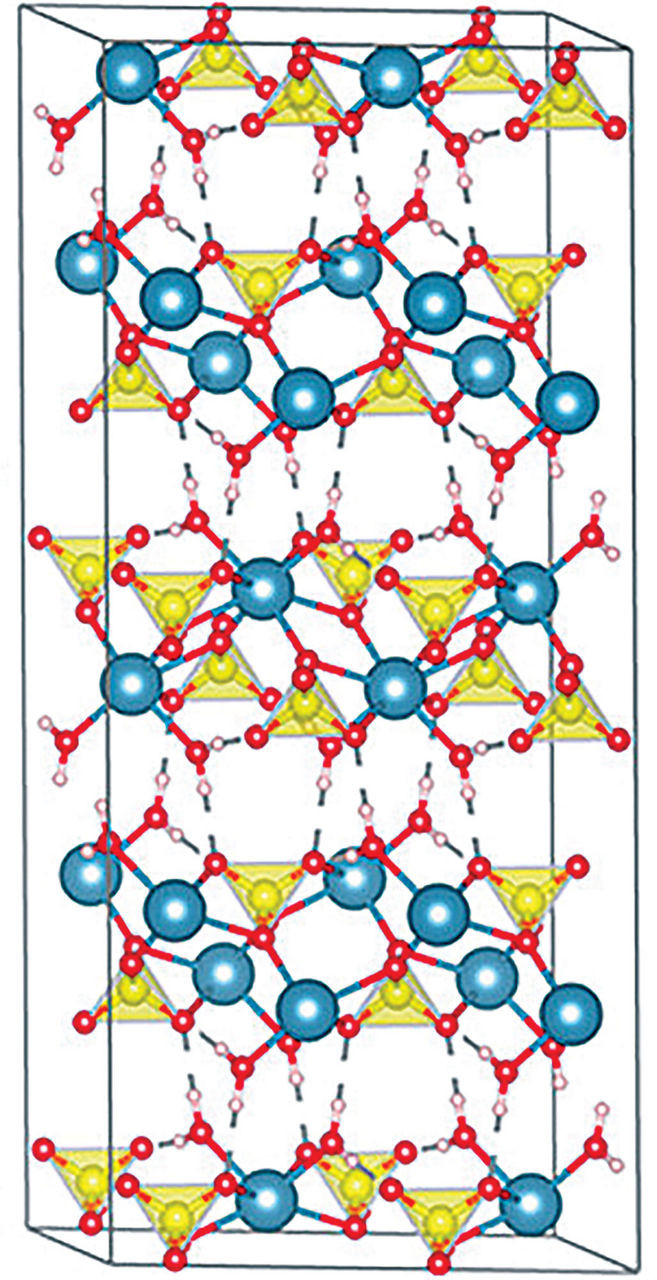 Gypsum crystal structure showing calcium, sulfur, oxygen, and hydrogen atoms.
Gypsum crystal structure showing calcium, sulfur, oxygen, and hydrogen atoms.
Crystallographer Alexander Van Driessche, from the National Center for Scientific Research’s Institute of Earth Science, who collaborated with García-Ruiz on studying the Naica crystals, explains that mineral stability is temperature-dependent. Anhydrite (CaSO4) is more stable at temperatures above approximately 58 °C, while gypsum is more stable – and less soluble – below this threshold.
Initially, as the magma-heated waters permeated the caverns, anhydrite deposits began to form. Over millennia, the water gradually cooled. As the temperature dipped below 58 °C, the anhydrite started to dissolve, and gypsum crystals began to nucleate and grow. The dissolving anhydrite continuously replenished the subterranean solution with just the right amount of calcium and sulfate, maintaining a state of slight supersaturation. These conditions were ideal for the exceptionally slow growth of gypsum crystals over vast stretches of time (Geology 2007, DOI: 10.1130/G23393A.1).
Whether in a laboratory or a natural cave, all crystals originate from nucleation. This is the process where molecular building blocks arrange themselves around a minute foundational form and begin to enlarge. For instance, in our atmosphere, solid particles act as nucleators for ice crystals that eventually fall as snowflakes. In ionic solutions, high supersaturation levels encourage the nucleation of numerous crystals, while low levels promote fewer nucleation sites but greater crystal growth. The Cave of Crystals offered the perfect balance, allowing a select few crystals to nucleate and, crucially, to develop into gigantic proportions.
Van Driessche and his colleagues, captivated by the Naica crystals, conducted laboratory experiments to study gypsum nucleation. Their findings revealed an unconventional nucleation mechanism: gypsum crystals develop from nanoclusters of CaSO4 that coalesce, rather than forming from a traditional tiny gypsum crystal as previously assumed (Science 2012, DOI: 10.1126/science.1215648).
Related: Learn more about the superslow growth of giant crystals.
Understanding the fundamental processes of gypsum crystal formation has implications that extend beyond the Mexican caves. Van Driessche and García-Ruiz believe this knowledge could aid in preventing unwanted mineral growth on equipment in desalination plants or in understanding gypsum formation on Mars. However, their scientific curiosity wasn’t limited to nucleation; they also sought to understand how the Naica beams achieved their immense size.
The Secret of Size: Infinitesimal Growth Over Immense Time
The Cave of Crystals isn’t the only cavern beneath the mountain near Naica containing gypsum crystals. The same mineral-rich waters permeated other underground chambers in the vicinity. However, the crystals in these adjacent caves never reached the astonishing dimensions found in the Cave of Crystals. Van Driessche and García-Ruiz concluded that, beyond the ideal initial conditions for crystal formation, a precisely gradual cooling rate was essential for the crystals to attain their mammoth sizes.
 A person explores the Cave of Swords, filled with shorter, sword-like gypsum crystals.
A person explores the Cave of Swords, filled with shorter, sword-like gypsum crystals.
The Cave of Swords, located at a shallower depth of 120 meters within the mine, provides a stark contrast. As its name suggests, its walls are densely covered with shorter gypsum crystals, resembling medieval swords, reaching up to 2 meters in length. In the deeper Cave of Crystals, the water cooled at a much slower pace than in the Cave of Swords. This gradual cooling over millennia resulted in fewer gypsum crystals nucleating, but those that did were given the time and resources to grow to extraordinary sizes, according to Van Driessche. Conversely, the faster temperature drop in the Cave of Swords led to a greater number of nucleation events, resulting in a multitude of smaller crystals. This difference serves as a “textbook example of crystal nucleation and crystal growth,” he notes.
Because the water temperature in the Cave of Crystals remained within the critical transition zone between anhydrite and gypsum for an extended period, the crystals grew continuously, undisturbed and undiscovered, for an immense duration. But precisely how long? Determining their age proved challenging. The crystals’ exceptional purity meant that isotope-dating techniques, reliant on trace elements like uranium, could only offer rough estimates. Instead, armed with crystal and water samples from the Naica mine, Van Driessche, García-Ruiz, and their colleagues embarked on meticulously measuring crystal growth rates in the laboratory.
The layered structure of gypsum crystals – calcium sulfate layers interspersed with double layers of water molecules – simplified this task. The hydrogen bonds between the water layers are easily broken, allowing flakes to be readily separated from the crystals, providing pristine, flat surfaces for growth studies, explains Van Driessche.
“When I entered the first time, after the first couple of minutes of stupor, I burst out laughing. I was euphoric.”
Juan Manuel García-Ruiz, crystallographer, University of Granada
After immersing the pristine gypsum crystal samples in water from the mine for approximately 24–48 hours, the team measured the increase in height of the flat surfaces using phase-shifting interferometry, a light-based technique capable of detecting surface growth rates as minute as 10–5 nm/s. By conducting measurements at various temperatures, the scientists could estimate the growth rates of the Naica crystals within their formation temperature range, approximately 54 to 58 °C. Using this data, they calculated the time needed for a 1-meter thick crystal beam to grow at 55 °C.
The result was astonishing, even considering the expected slow growth. Such crystals would have taken nearly 1 million years to reach that size (Proc. Natl. Acad. Sci. U.S.A. 2011, DOI: 10.1073/pnas.1105233108). Van Driessche likens this growth rate to adding the thickness of a sheet of paper every 200 years.
A Fragile Future: Drained and Delicate
If the Cave of Crystals had remained submerged, there’s no knowing the ultimate size the crystals might have attained. The current conditions at that depth, with a water temperature of around 55 °C, are still conducive to gypsum crystal formation and growth, according to Van Driessche. Small ponds within the mine even contain newly forming gypsum crystals. However, years of mining operations in Naica have artificially lowered the water table to facilitate access to lead, zinc, and silver deposits. While draining the cave revealed the giant crystals, it also exposed them to a drastically different, and potentially damaging, environment.
During the operation of the Naica mine, Industrias Peñoles pumped water out of the mountain at a rate equivalent to filling an Olympic-sized swimming pool every 40 minutes, creating an artificial lake near Naica, Van Driessche notes. As the water level receded, more of the caves and galleries became accessible to air – and to humans. The Cave of Crystals, due to its depth and relatively recent discovery, escaped the extensive looting that occurred in the shallower Cave of Swords, discovered in 1910, where collectors removed many of the largest and most aesthetically pleasing crystals.
Peñoles implemented strict access controls to the Cave of Crystals, aimed at protecting both the crystals and visitors. The conditions inside the cave push human physiology to its limits. The temperature hovers around 50 °C (122 °F), with a relative humidity exceeding 90%. In such an environment, sweating provides no cooling effect, making prolonged exposure dangerous.
“I just want to see them once more.”
María Elena Montero-Cabrera, researcher, Center for Research in Advanced Materials
Navigating the cave is also perilous, explains María Elena Montero-Cabrera, a researcher at the Center for Research in Advanced Materials in Chihuahua. Researchers had to carefully climb over condensation-slick gypsum spears to explore the cave, taking extreme caution to avoid getting lost or falling, as rescue operations would be incredibly complex and hazardous.
Given the extreme conditions, researchers could only spend brief periods, typically 10–15 minutes, inside the cave at a time, Montero-Cabrera recounts. The cave is sealed off from the rest of the mine by two sets of doors, isolating the crystals from the external mine environment and maintaining a more tolerable temperature and humidity in the antechamber for human safety. Prior to each entry, a medical check was mandatory to ensure visitors were fit enough to endure the cave’s climate.
Beyond the challenges for researchers, these harsh conditions also pose a threat to the crystals themselves. The largest beams are estimated to weigh 40–50 metric tons, and without the buoyant support of water, they are at risk of cracking under their own weight. Gypsum is a relatively soft mineral, rated 2 on the Mohs hardness scale (talc is 1, diamond is 10). The passage of human feet has already visibly worn a darkened path into the crystals on the cave floor, Van Driessche observes.
Related: Discover more about the dehydration risks faced by the giant crystals in the Mexican cave.
To devise effective preservation strategies for future generations, Montero-Cabrera and her research team in Chihuahua investigated the impact of the drained environment on the crystal surfaces. In a year-long laboratory experiment, they exposed samples of the Naica crystals to various gaseous and liquid environments to identify potential changes or contaminants that could compromise the gypsum’s long-term integrity and appearance.
Their findings indicated that the crystals fared slightly better in a liquid environment, while gaseous environments presented a risk of dehydration. Specifically, they detected bassanite, the dehydrated form of calcium sulfate, on the surface of several gypsum crystals (Cryst. Growth Des. 2018, DOI: 10.1021/acs.cgd.8b00583). This suggests that the crystals’ appearance will gradually change over time, reinforcing the conclusion that removing crystals from the sealed cave is not a viable preservation method, according to Montero-Cabrera.
An Uncertain Future for a Crystal Wonderland
Research into the giant crystals of Naica has gradually decreased as many fundamental questions have been addressed. Mining operations closed access to the cave around 2015 due to a leak that flooded the mine faster than pumps could manage. Although the water level in the mine has since risen, it’s unclear if it has reached the Cave of Crystals.
 A person stands inside a geode with transparent gypsum crystals similar to those in the Cave of Crystals.
A person stands inside a geode with transparent gypsum crystals similar to those in the Cave of Crystals.
Montero-Cabrera notes that recent reports suggest mining activity might resume through a different entrance, potentially allowing researchers renewed access to the cave. Whether the cave will flood again remains uncertain. In the meantime, researchers are turning their attention to other captivating gypsum deposits worldwide to further their understanding of gypsum crystallization and growth.
Related: Explore the genesis of gypsum crystals and their formation processes.
For instance, García-Ruiz and Van Driessche are currently studying crystals found within an 8-meter-long geode in Pulpí, southern Spain. The gypsum crystals there, while smaller, are more transparent than those in the Mexican Crystal Cave, prompting the team to investigate the reasons behind these morphological differences. In addition to understanding these variations, García-Ruiz hopes to further refine his age estimate for the Naica crystals.
Although Montero-Cabrera has shifted her research focus, she expresses a strong desire to return to the Cave of Crystals if it becomes accessible again. “I just want to see them once more,” she says, echoing the sentiment of many who have been touched by the cave’s surreal beauty.
Until then, the giant crystals remain isolated – a concealed, extraordinary wonder awaiting an unknown future.
Emma Hiolski is a freelance writer. A version of this story first appeared in ACS Central Science: cenm.ag/crystalcave.
CORRECTION: On Feb. 12, 2019, a photo caption was updated to reflect that the gypsum crystals grew over many millennia.
Sign up for C&EN’s must-read weekly newsletter.
Chemical & Engineering News
ISSN 0009-2347
Copyright © 2025 American Chemical Society